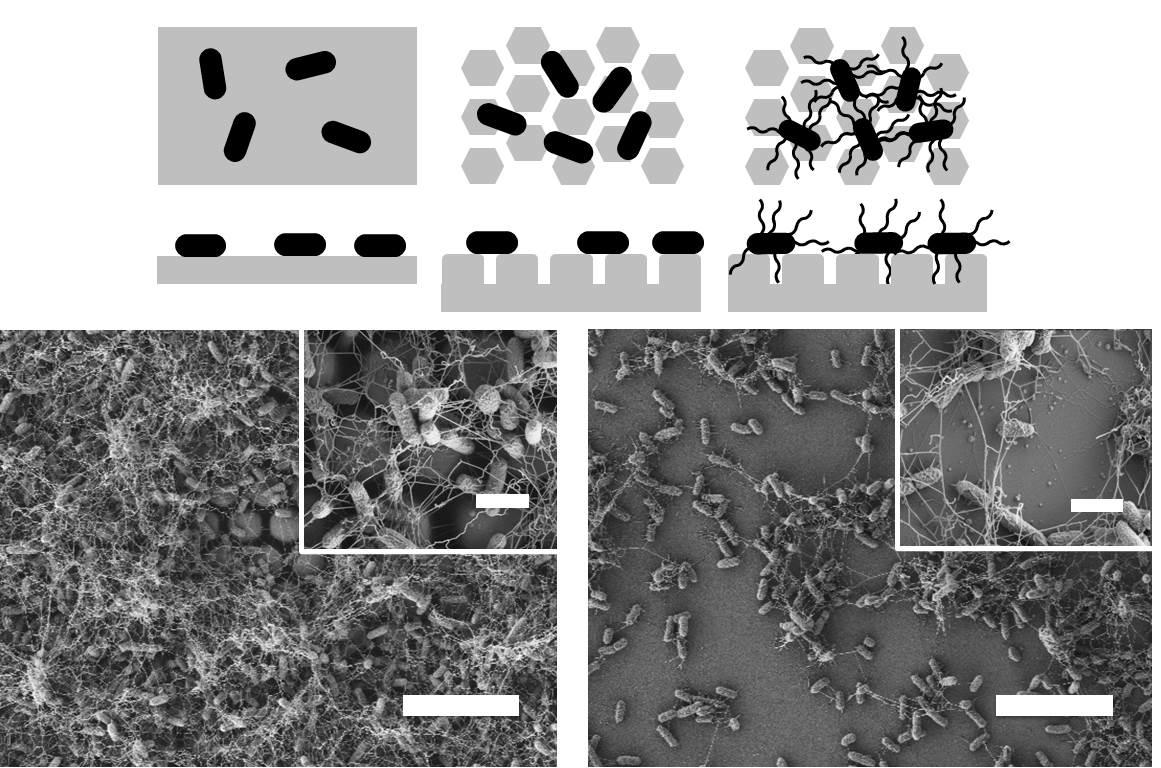
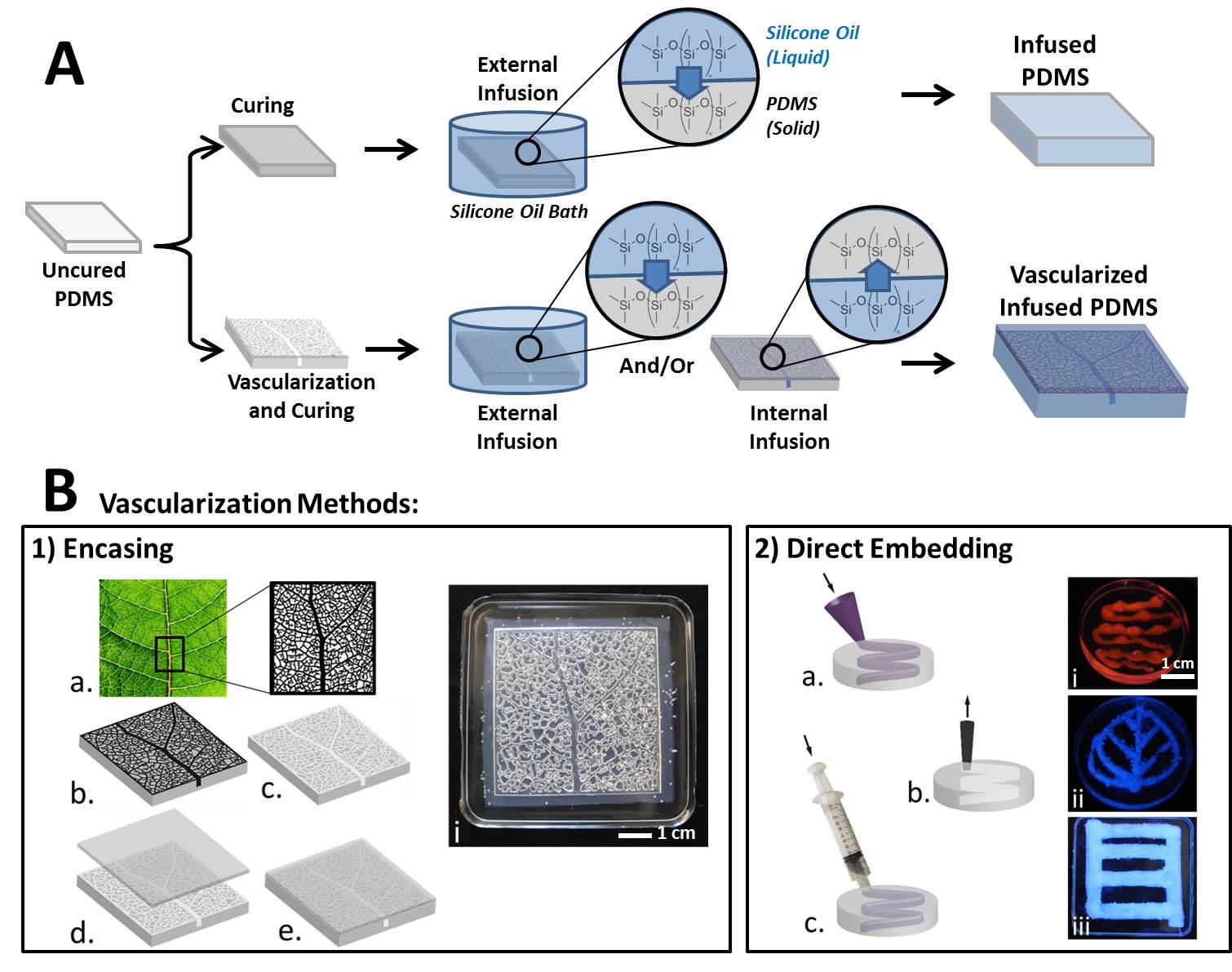
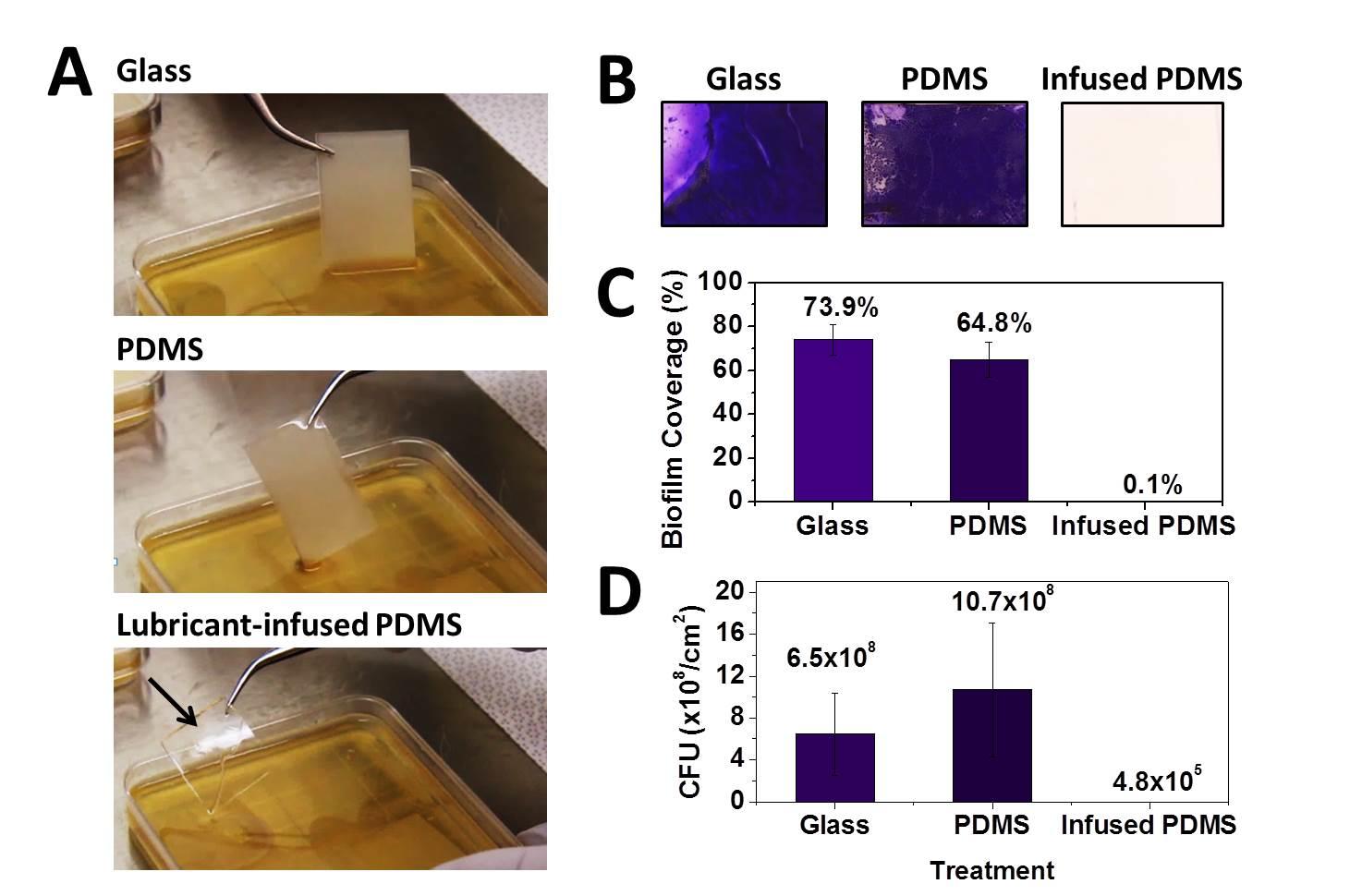
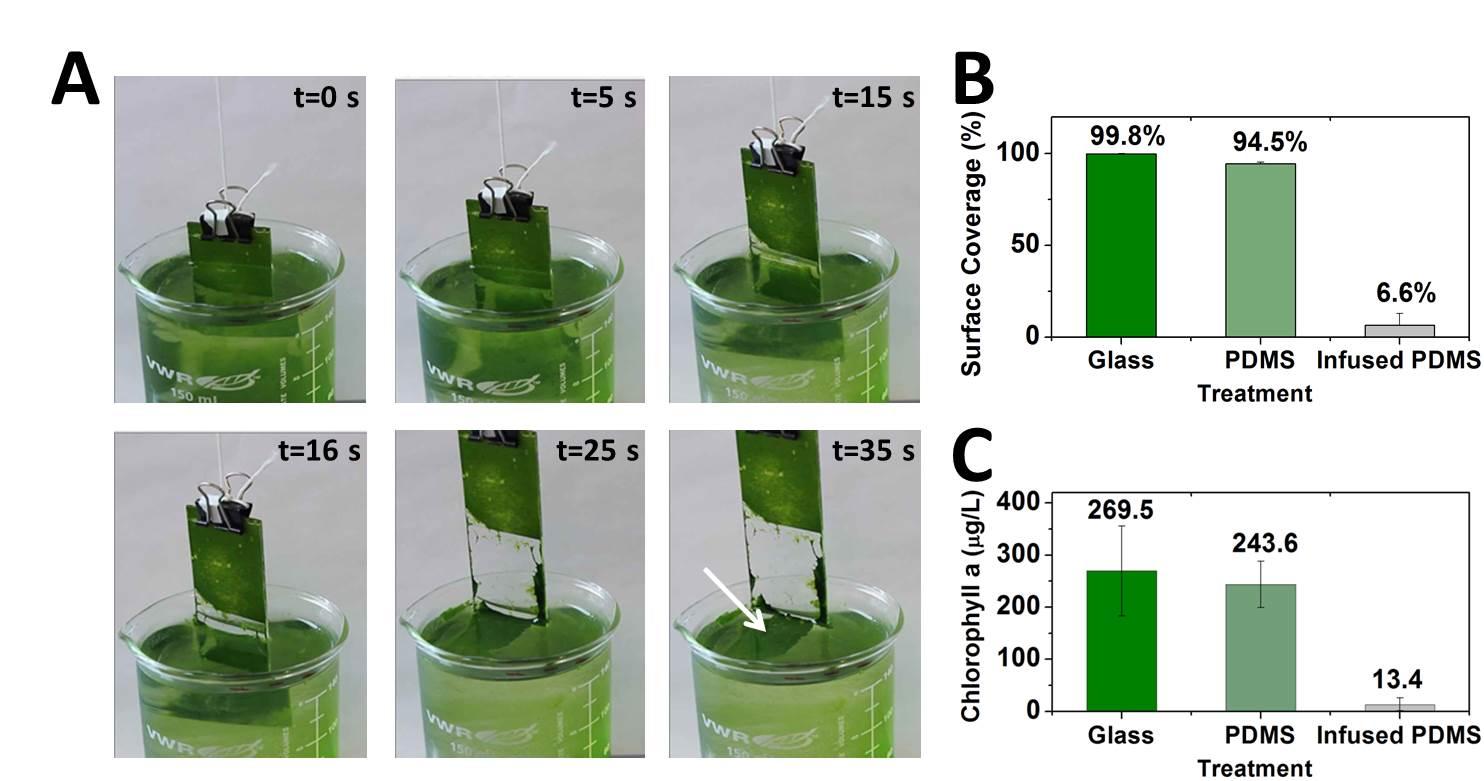
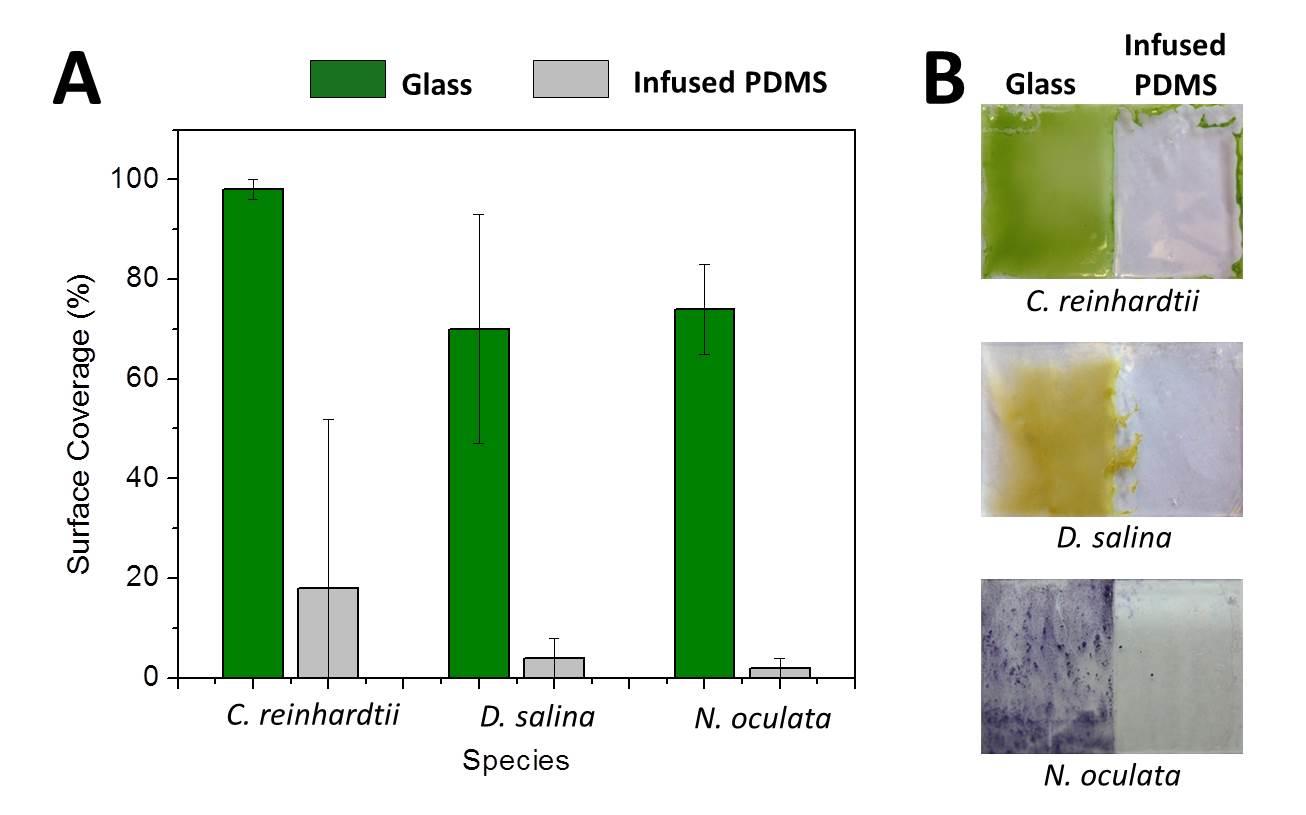
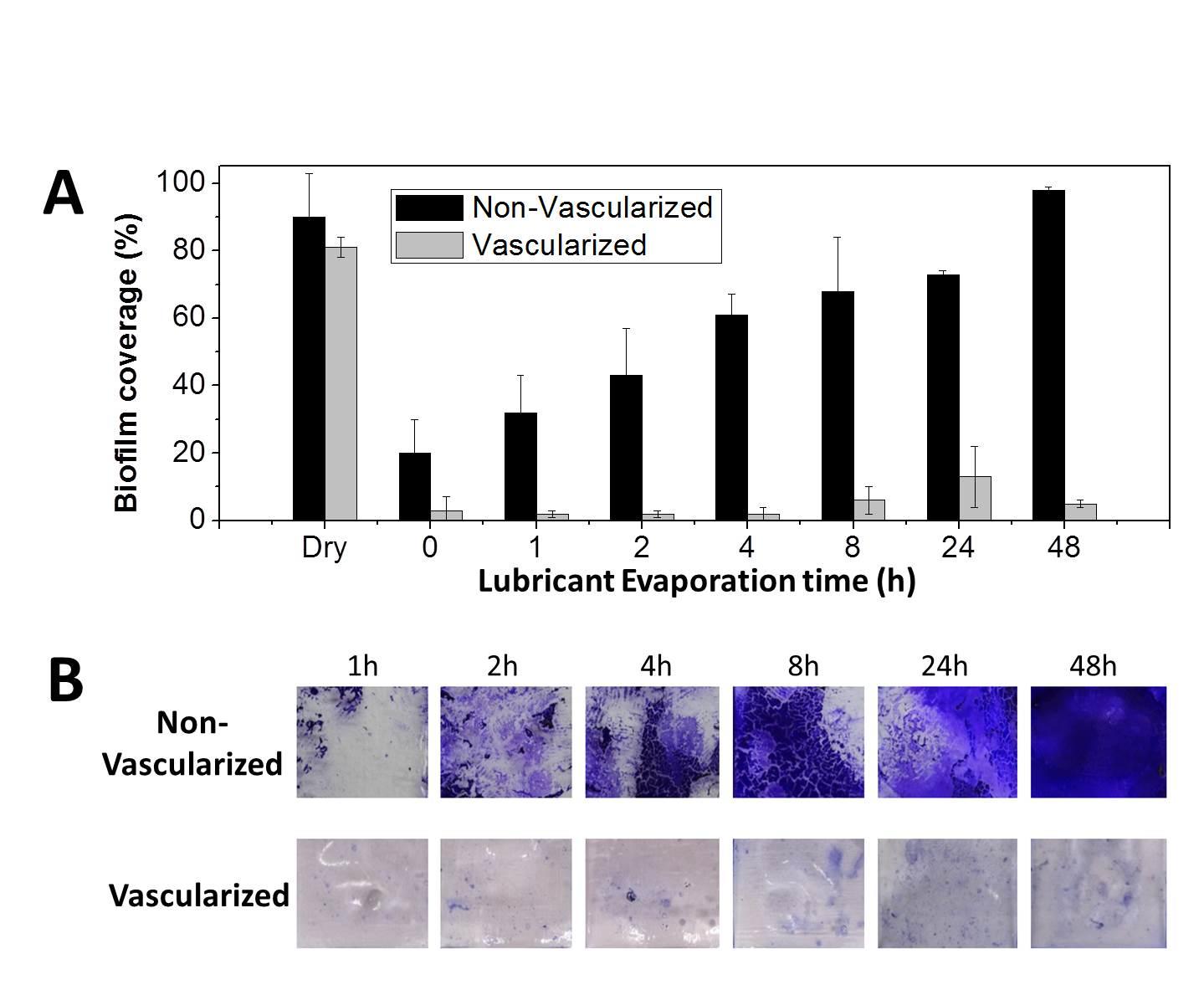
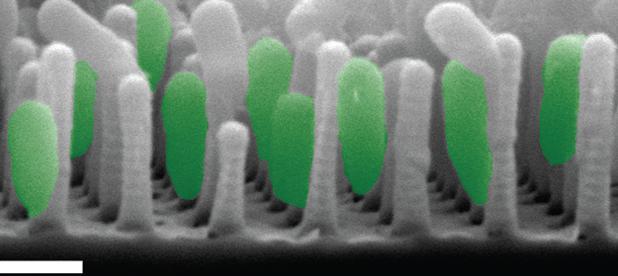
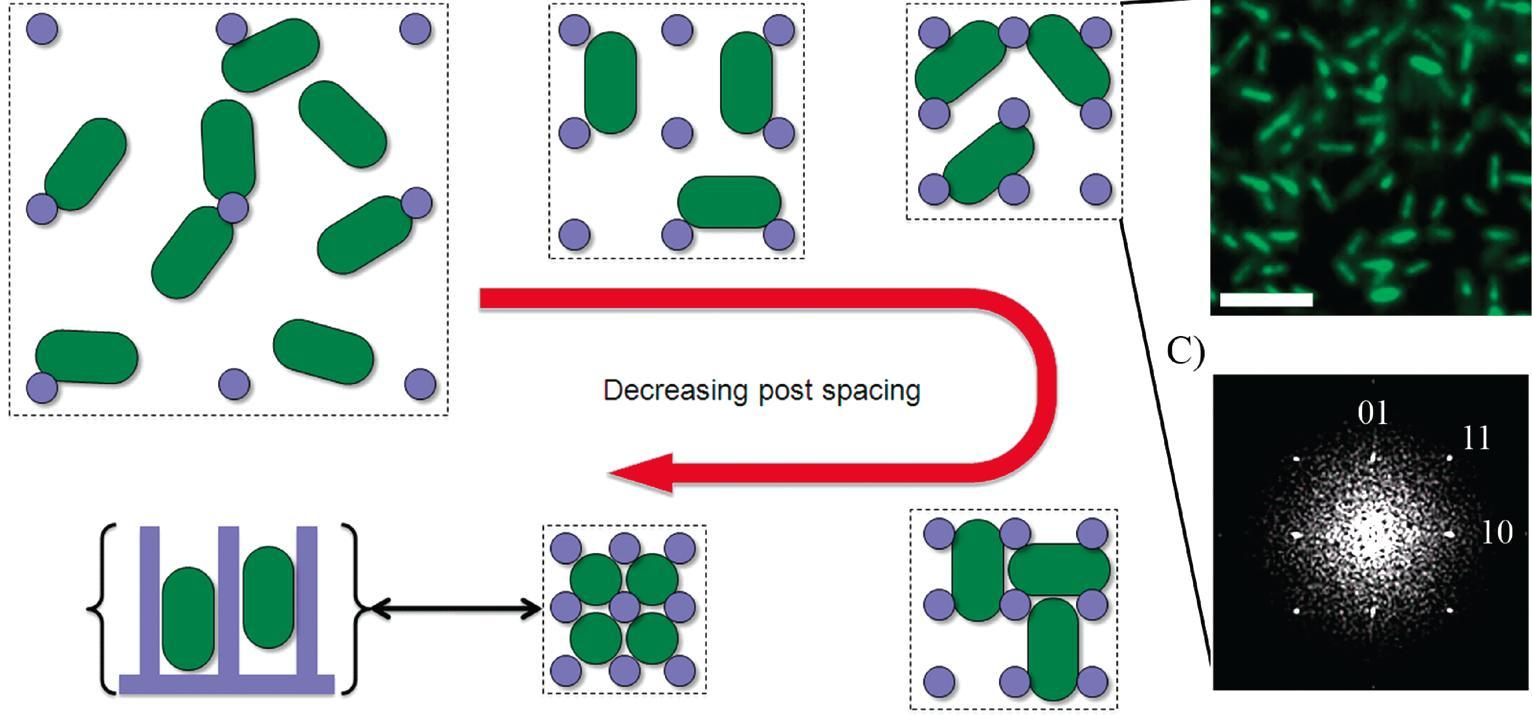
Cells of all types take cues from the surfaces they encounter in their environment. The cells extract any or all of the mechanical, chemical, spatial, and even temporal information from the surface, but which features they read and how they integrate and translate them into specific behavioral responses is still almost a black box.
Bionanointerfaces
Projects
Vascularized self-lubricating surfaces
We have recently developed a technique for fabricating nanostructured surfaces that enables us to choose and independently vary any combination of geometry, spacing, stiffness, and chemistry, and are using it to analyze how surface features direct bacterial assembly, mammalian cell development, and marine invertebrate settlement at a level of systematic detail that was previously not possible.
As our insight into the cell-nanosurface interface develops, the same fabrication technique will enable us to design and construct surfaces that encode instructions for cell behavior for purposes ranging from anti-biofilm coatings to neural chips to organ development scaffolds.
- 1 of 2
- »
Clinging to crevices, E. coli thrive, Harvard press release, April 10, 2013.